PITTSBURGH-Carnegie Mellon University scientist Chien Ho and his
colleagues have developed a promising tool that uses magnetic
resonance imaging (MRI) to track immune cells as they infiltrate a
transplanted heart in the early stages of organ rejection. This
pre-clinical advance, described in an upcoming issue of the
Proceedings of the National Academy of Sciences (PNAS), ultimately
could provide a noninvasive way to detect transplant rejection in
patients.
"We have reported for the first time the ability to monitor single
immune cells in a live animal using MRI. This could revolutionize the
management of transplant patients," said Ho, professor of biological
sciences at the Mellon College of Science.
"Successful translation of this work to the clinic ultimately will
reduce the number of biopsy procedures and should greatly improve the
quality of life for cardiac transplant patients, especially children,"
added Ho, who directs the Pittsburgh NMR Center for Biomedical
Research. "Perhaps most importantly, this advance will allow doctors
to provide highly personalized care that could prevent transplant
rejection."
Organ transplantation is the preferred clinical approach to treat
end-stage organ failure, but transplant patients face a lifetime of
immunosuppressive therapy and the risk of losing the new organ due to
rejection. Physicians typically monitor patients for organ rejection
following a heart transplant by performing frequent heart biopsies for
the first year. Heart biopsies are invasive procedures that involve
threading a catheter through the internal jugular vein to the heart's
right ventricle and snipping out several tiny pieces of tissue. A
pathologist then tests the tissue to identify the presence of immune
cells (such as macrophages) as well as other pathological changes in
the transplanted heart tissue that indicate the graft is being
rejected by the body's immune system.
These procedures are costly, uncomfortable and must be repeated
annually to monitor and treat any rejection. Biopsies also are
problematic, according to Ho, because they do not look at the whole
organ. By only sampling several small areas, a biopsy may miss the
area of the transplanted organ where immune cells are gathering-one of
the first signs of rejection.
Ho's novel approach investigates transplant rejection non-invasively
by observing macrophage accumulation in heart tissues using MRI.
"We were able to use MRI to visualize individual macrophages. By
tracking individual cells, we also were able to observe, for the first
time, that rejection progresses from the outside of the heart to the
inside," said Ho. "Up to now, this phenomenon hasn't been observed in
pre-clinical or clinical research because biopsy samples are very
limited in location and size."
The reported findings also have broader implications for biology and
medicine, according to Ho.
"We now have the ability to visualize non-invasively and with
sensitivity individual cells and their movement to targeted sites. Our
new approach offers almost unlimited potential for monitoring cell
therapies, such as those using stem cells, and for tracking cellular
and developmental processes," Ho said.
For the research reported in PNAS, Yijen Wu, research biologist at the
Pittsburgh NMR Center for Biomedical Research, tagged macrophages with
nanometer (USPIO)- or micrometer (MPIO)-sized paramagnetic iron oxide
particles, which are very sensitive to the magnetic fields used during
MRI. Wu injected the MPIO or USPIO particles into rats that had
received heart transplants three days earlier. Macrophages, which
typically ingest foreign materials inside the body (bacteria, for
example), incorporated the particles. Using MRI, the researchers then
are able to track tagged macrophages that infiltrate transplanted
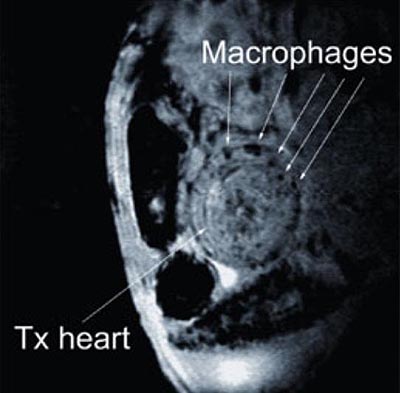
hearts. The locations of the tagged macrophages are highly defined and
appear circular in shape, said Wu. (See image below.) This finding
indicates that the new, real-time tracking method is very good at
pinpointing exactly when and where rejection is taking place.
Chien Ho and his colleagues are using a heterotropic cardiac
transplantation model in rats (above) to develop a non-invasive, MRI-based
method to monitor organ rejection. NMR Center scientists label immune
cells with MRI contrast agents and track their accumulation at the
rejecting graft (dark spots), which is an early sign of organ
rejection. (Photo courtesy of Chien Ho, Carnegie Mellon University)
The researchers used a heterotropic heart model to study organ
rejection. In this model, a rat receives a second functional heart,
which is grafted into its abdomen. The rat's native heart functions
normally. In this way, the researchers can study how a transplanted
heart changes through sequential stages of rejection while the rat
stays healthy. This aspect of the research was conducted primarily by
Qing Ye, a research biologist at the Pittsburgh NMR Center for
Biomedical Research.
Ho's team at the Pittsburgh NMR Center for Biomedical Research is now
pursuing research using larger animal models. They are collaborating
with researchers at the University of Pittsburgh School of Medicine,
including Dr. David Cooper, professor of surgery in the Thomas E.
Starzl Transplantation Institute; Dr. Jeffrey Teuteberg, assistant
professor of medicine at the Cardiovascular Institute, Heart
Failure/Transplantation; and Dr. Fernando Boada, associate professor
in the Department of Radiology.
The research is funded by the National Institute of Biomedical Imaging
and Bioengineering, the National Center for Research Resources, the
National Heart, Lung and Blood Institute, as well as the Health
Research Formula Funds of the Pennsylvania Commonwealth University
Research Enhancement Tobacco Settlement.
Established in 1986 and funded continuously since 1988 by the National
Institutes of Health, the Pittsburgh NMR Center for Biomedical
Research is dedicated to enhancing molecular, cellular and functional
imaging using small animals. The center, sponsored jointly by Carnegie
Mellon and the University of Pittsburgh, makes major contributions to
the rapidly growing field of nuclear magnetic resonance in biology and
medicine.
The Mellon College of Science at Carnegie Mellon develops innovative
research and educational programs in a range of scientific,
interdisciplinary areas. For more information, visit www.cmu.edu/mcs.
-- by Lauren Ward, Carnegie Mellon
University 412-268-7761
Dr. Chien Ho
Education: Williams College (BA), Yale University (Ph.D.),
Massachusettes Institute of Technology (postdoctoral training)
Honors: John Simon Guggenheim Fellowship; Elected to membership in the
Academia Sinica, Taiwan; named Alumni Professor of Biological Sciences
at CMU; National Heart, Lung and Blood Institute MERIT award
Professonal/academic achievement: on advisory committees at various
universities and Institutions such as NIH, NSF, Stanford University,
Univ. of Pennsylvania, Baylor College of Medicine, etc.
Invited to give talks at national and international scientific
meetings.
|